.jpg)
.jpg)
Since the legalization of e-cigarettes in April 2019, the UAE, particularly Dubai, has seen rapid growth in the vaping sector, drawing many companies into the market.For vape brands and manufacturers, understanding local regulations, taxes, and compliance requirements is essential before entering the market.
This guide provides a concise overview of the UAE’s e-cigarette regulations and outlines the key steps for compliant market entry.
Table of Contents - Market Snapshot - Regulatory Framework - Key Regulatory Agencies - Product and Packaging Requirements - Vape Products Registration Fees, Procedures |

The UAE vaping market is valued at approximately USD 300 million, with over one million active users. However, growth is expected to decelerate in 2025, with the annual expansion rate projected to fall below 16%, signaling a gradual market slowdown.
As the largest distribution hub for e-cigarettes in the Middle East, Dubai has long served as a strategic testing ground for vape brands, due to its relatively relaxed regulatory environment, high-spending consumer base, and a large, diverse population of open-minded young users. These conditions offer advantages for product validation and market entry.
At Dragon Mart, the largest wholesale trading hub for vape products in the UAE, compliant e-cigarettes are sold openly, but with careful inquiry, irregularly imported products can still be found. Although the number of vape shops has visibly increased in recent years, foot traffic has become noticeably lighter. As distribution networks across the Middle East have matured, many buyers now prefer localized direct channels over Dubai. This shift has gradually eroded Dubai’s former role as the region’s central distribution hub, further contributing to the market’s slowing momentum.
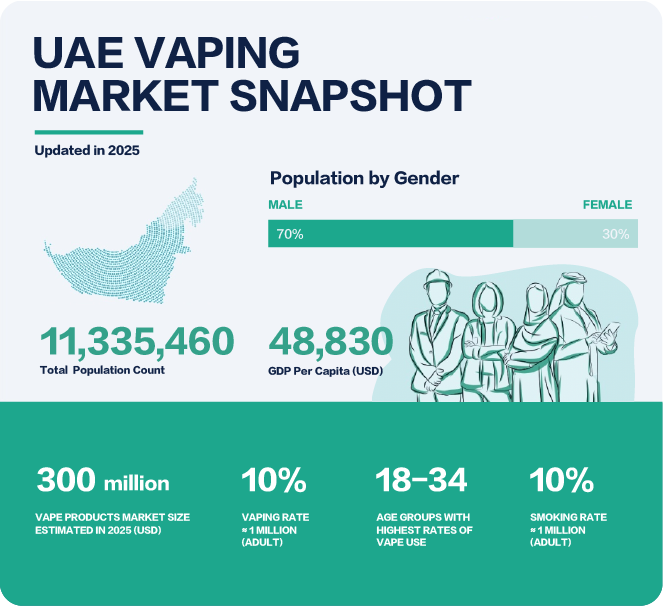
Population: As of 2025, the UAE's population is approximately 11.3 million. The majority of the population is concentrated in the three largest emirates: Dubai, Sharjah, and Abu Dhabi, which together account for about 85% of the total population. specifically, the population of Abu Dhabi is around 3.8 million in 2024, Dubai's population is about 3.5 million, and Sharjah's population is approximately 1.8 million. The gender distribution in the UAE shows a higher proportion of males, with approximately 70% of the population being male and 30% being female. Additionally, expatriates make up around 88.5% of the total population.
Smokers: In the United Arab Emirates 10% of the adult population are smokers, totaling approximately 1million, with 15.5% of men and only 2.5% of women currently smoking.
Vapers: The rate of e-cigarette usage among adults is over 10%. The usage is higher among males than females, and the distribution is more prevalent in urban areas such as Dubai and Abu Dhabi. E-cigarette usage is most common among young adults aged 18-34.
Market Size: The UAE e-cigarette market is expected to reach approximately USD 300 million by 2025. This growth is driven by government regulatory relaxation, increasing consumer demand for healthier tobacco alternatives, and the diversity of products available in the market.
GDP: The GDP per capita in the UAE was USD 48,830 in 2023. Abu Dhabi's GDP is about USD 310 billion, Dubai's is about USD 100 billion, and Sharjah's is about USD 40 billion.
What Regulations do E-Cigarette Companies Follow in the UAE?
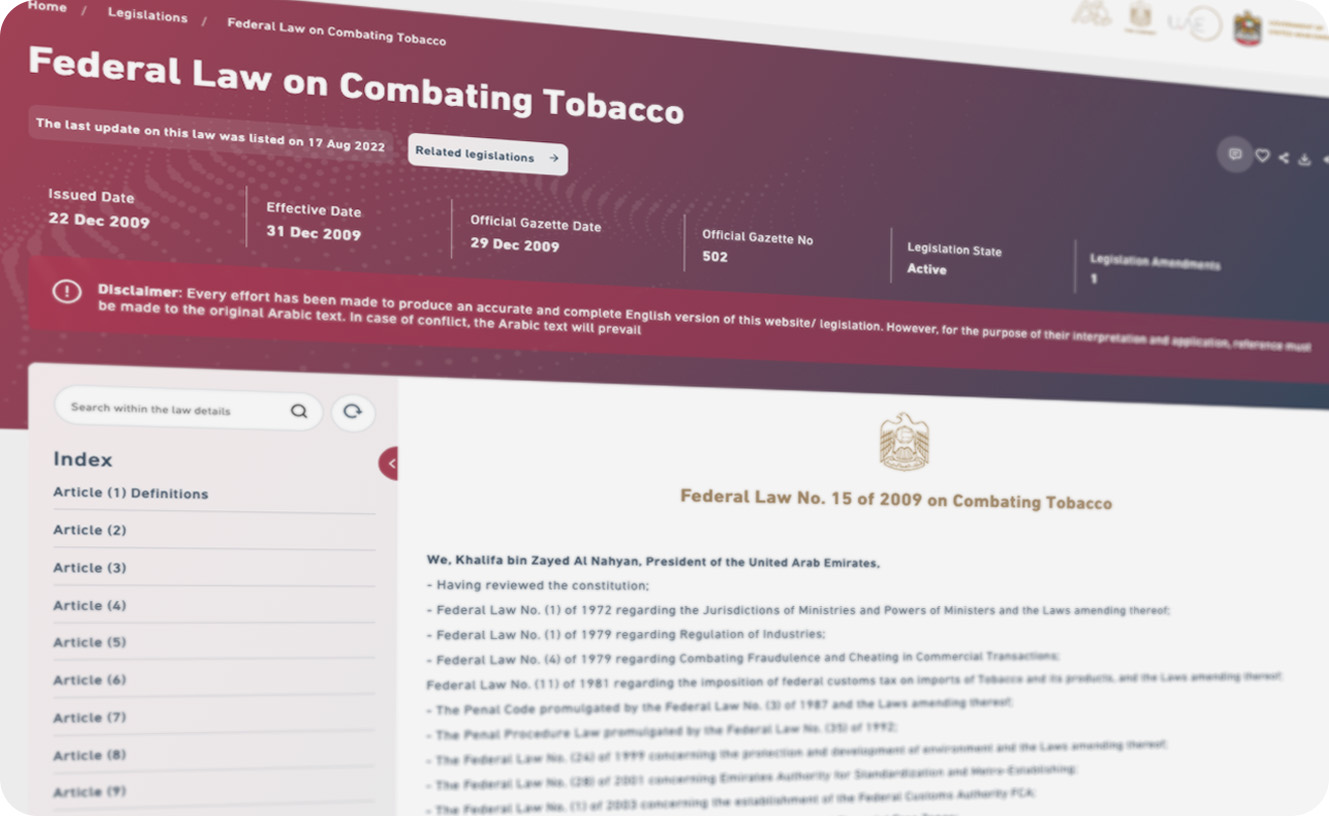
Federal Law No. 15 of 2009
Governs tobacco control, including e-cigarettes. It prohibits sales to minors, restricts advertising, and regulates public use.

UAE.S 5030:2018 Standard
Specifies safety, ingredient, packaging, and labeling requirements for e-liquids and devices.
All e-cigarette products in the UAE must obtain ECAS certification, carry FTA-issued digital tax stamps, and be sold by entities holding valid DED trade licenses and excise registrations.
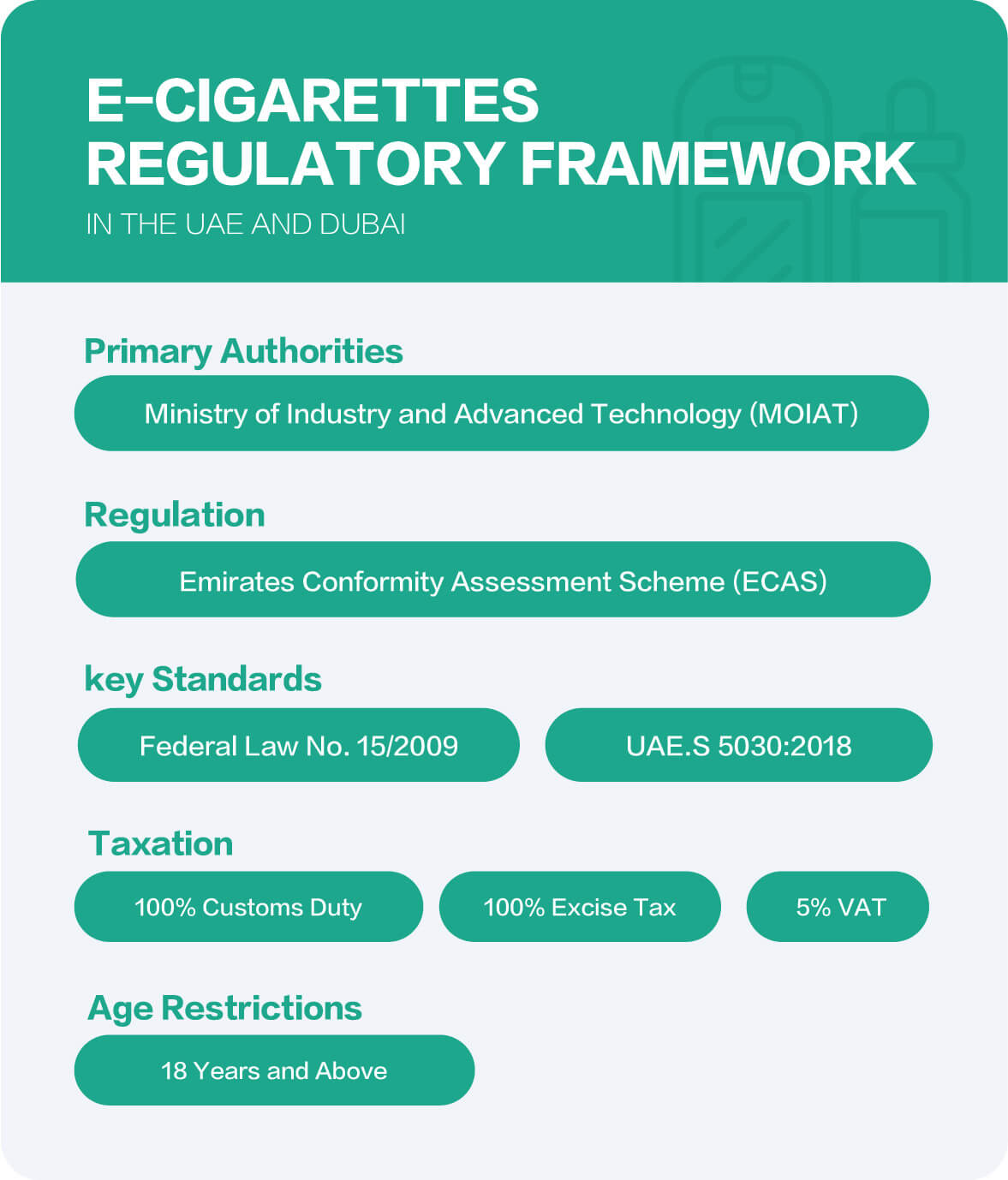

Regulatory Agencies
Ministry of Industry and Advanced Technology (MoIAT)
MoIAT, established in July 2020, plays a crucial rolein advancing the UAE’s industrial sector by developing national policies, promoting innovation, and ensuring sustainability across various industries. In the e-cigarette industry, MoIAT is the primary regulatory body responsible for overseeing the conformity assessment and certification of products to ensure they meet health, safety, and environmental standards.
The main functions include the following:
- Formulating standards that cover the safety, quality, and environmental impact of products, including e-cigarettes.
- Overseeing the Emirates Conformity Assessment Scheme (ECAS) and handling the issuance of conformity certificates for products (a role previously managed by ESMA) to ensure they meet the UAE's safety and quality requirements.
Emirates Authority for Standardization and Metrology (ESMA)
Founded in 2001, ESMA sets and regulates standards for various products in the UAE. Following the July 2020 Cabinet restructuring, ESMA was integrated into MoIAT, and its functions were taken over by MoIAT to streamline regulatory processes.
Federal Authority for Identity, Citizenship, Customs & Port Security (ICP)
ICP manages the import and customs processes for e-cigarette products entering the UAE.
The main functions include the following:
- Overseeing the importation process to ensure only compliant products enter the country.
- Implementing customs duties and excise taxes on e-cigarette products.
Ministry of Health and Prevention (MOHAP)
MOHAP is responsible for regulating the health aspects of e-cigarettes, ensuring these products do not pose undue health risks to consumers.
The main functions include the following:
- Conducting health impact assessments and research on e-cigarettes.
- Issuing guidelines and warnings about the health implications of e-cigarette use.
Federal Tax Authority (FTA)
FTA manages the implementation of Digital Tax Stamps (DTS) for e-cigarettes to ensure tax compliance.
The main functions include the following:
- Implements Digital Tax Stamps (DTS) for e-cigarettes.
- Registers companies and processes DTS applications.
- Ensures compliance with excise tax and VAT regulations.
Department of Economic Development (DED)
DED in each emirate are responsible for business licensing and economic regulations concerning the sale and distribution of e-cigarettes.
The main functions include the following:
- Issuing trade licenses for businesses involved in the e-cigarette industry.
- Monitoring commercial activities to ensure compliance with local economic laws and regulations.
Gulf Cooperation Council (GCC) Standardization Organization (GSO)
GSO works on creating unified standards for e-cigarette products across GCC member states, including the UAE.
The main functions include the following:
- Developing and harmonizing regulations and standards for e-cigarettes.
- Facilitating cross-border trade compliance within GCC countries.
Product and Packaging Requirements
Product Definition
Vape Products (UAE.S 5030:2018, Sec. 3.6)
Electronic devices and its accessories which operate through batteries, and can be used to heat the e-liquid to be vaporized, inhaled and exhaled by mouth as a smoke simulation, and they are free of tobacco. A typical electronic cigarette consists of: the battery that is used to heat the solution tank containing the so-called "electronic liquid" that may be in a disposable cartridge or a tank that can be refilled from external cartridges.
Electronic Liquid (UAE.S 5030:2018, Sec. 3.7)
A liquid formula (or gel) in the Electronic Vape Products or in a replaceable cartridge (which may or may not contain nicotine) and some other additives such as distilled water, solvents, vegetable glycerin and propylene glycol.
General requirements
Devices must meet GSO standards, including specific safety standards for batteries and electrical appliances.
1. The manufacturing facilities must comply with the quality management system requirements outlined in UAE.S GSO ISO 9001.
2. Electronic and electrical devices must meet the safety requirements set forth in UAE.S 5030:2018 or an equivalent standard, including drop tests, contact temperature tests, and electromagnetic compatibility (EMC) testing.
3. Devices must comply with the Cabinet Decree No. 10 of 2017, which governs the UAE system for restricting hazardous substances in electrical and electronic equipment (RoHS).
4. Devices intended for use within a specific voltage range (low voltage or LVD) must meet the standards defined in UAE.S GSO IEC 60335-1.
5. Batteries, accumulators, secondary cells, and chargers must comply with the following applicable standards:
• UAE.S IEC 62133
• UAE.S IEC 61960
• UAE.S GSO IEC 60086
• UAE.S GSO IEC 60950-1
6. Products and/or cartridges must comply with UAE.S 5030-2:2022 regarding child safety. This includes resistance to tampering by children, child-resistant mechanisms for opening and closing, and appropriate safety markings.
7. Products and cartridges must remain compliant with all applicable requirements of UAE.S 5030:2018 throughout the shelf life stated on the packaging.
8. No changes to the ingredients or characteristics of the product or cartridges are permitted without prior official approval from ESMA.
9. Products and cartridges may not be imported, manufactured, or marketed in the UAE without formal registration approval from ESMA.
10. Materials necessary for product and cartridge manufacturing—such as sugar used to replace losses during processing—are permitted only if they do not increase addictiveness or toxicity.
11. The product packaging or outer packaging must not include printed vouchers, discount offers, free samples, “buy one get one free” promotions, or any other economic incentives that may mislead consumers or promote impulsive purchases.
1. Manufacturing must comply with UAE.S 5030:2018.
2. Nicotine and solvents must be high purity and pharmaceutical grade, compliant with the European Pharmacopoeia.
3. The microbiological limits of nicotine must not exceed levels specified in the European Pharmacopoeia.
4. Maximum nicotine concentration: 20 mg/ml
5. E-liquid tanks: max 10 ml
6. Refill packages: max 50 ml
7. Packaging must be child-resistant, leak-proof, and not easily broken or damaged, in accordance with S/GSO ISO 8317.
8. Packaging must prevent leakage of e-liquid into the mouth during inhalation.
9. Ingredients must not pose health risks under intended use, except for nicotine and approved tobacco extracts.
10. Permitted moisturizers include food-grade, non-petroleum glycerol, 1,2-propylene glycol, 1,3-butylene glycol, and triethylene glycol with at least 99.5% purity. These must meet pharmaceutical-grade standards.
11. All additives must be food-grade and comply with the UAE standard UAE.S 192:2019
12. Flavoring substances may be used only if compliant with approved UAE food flavoring standards GSO 707:2023 (Historical Editions GSO 707:2021).
13. If allergenic or hypersensitivity-related substances are used, this must be clearly indicated on the label.
Prohibited Ingredients
Ingredients used in e-liquids shall not pose a health hazard, either as raw materials or under intended use conditions and concentrations, except for nicotine and permitted tobacco extracts.
- Vitamins or other additives that give the impression of health benefits or reduced risks
- Caffeine, taurine, or other performance/vitality stimulants
- Colored additives with emission-coloring properties
- Substances with CMR (carcinogenic, mutagenic, or reprotoxic) properties before or after use
- Legally prohibited substances (e.g., narcotics, hallucinogens, tranquilizers)
- Ethylene glycol, diethylene glycol, formaldehyde, acetaldehyde, acrolein, crotonaldehyde, acetone, acetylpropionyl, diacetyl, pentane-3,2-dione, and related ketones, long-chain paraben preservatives
- Acrylonitrile, benzene, 1,3-butadiene, isoprene, toluene
- 4‑amino‑diphenyl, 1‑amino‑naphthalene, 2‑amino‑naphthalene
- Ammonia
- Cinnamic compounds
- Respiratory allergens
- Heavy metal residues (lead, cadmium, mercury, chromium, nickel, iron, arsenic, tin)
- Polycyclic aromatic hydrocarbons, carbon monoxide, and tobacco nitrosamines (e.g., NNK, NNN)
- Mineral and vegetable oils or fats (e.g., olive oil)
Flavors
Under UAE law, the use of cinnamic compounds in e-cigarette products is prohibited. Consequently, all cinnamon-flavored e-liquids are banned, and the product category is assigned a regulatory status of “Some Restrictions.”
Labelling and Packaging requirements
The following information is for reference only. Please refer to the specific requirements as detailed in the UAE.S 5030:2018 document.
1. Product name and trade name
2. Dates of packaging – month and year
3. Production Batch number
4. Product ingredients – flavor used must be placed near the product name
5. Country of origin, manufacture or packaging
6. Health warning for products that contain nicotine
7. Warning against the sale to, and consumption by, minors
8. The official e-liquid warning
9. The following phrase:
للبیع في دولة الامارات العربیة المتحدة
(For sale in the United Arab Emirates)
E-liquids Warning
The packaging of e-liquids, regardless of nicotine content, is in both Arabic and English:
Warning in Arabic

Warning in English

Health Warning
Warning in Arabic

Warning in English
.png)
Health warnings must cover over 50% of the primary display surface, with English on the front and Arabic on the back.
Minors’ Protection Warning
The following warning must be placed on the package, with the written statement in both Arabic and English along with the symbol pictured below which should be a minimum of 1 cm x 1 cm:
Warning in Arabic

Warning in English

Information on the packaging must be clearly and legibly written in Arabic or English, with text colors that are distinctly different from the background. This information must be permanent and not removable or detachable from the packaging.
The following elements must not be included on the packaging:
1. Printed vouchers, discounts, two-for-one or free distribution offers
2. Elements creating an erroneous impression about its characteristics, health or therapeutic effects, hazards, or emissions
3. Names, symbols, images, or phrases that may violate public order
4. Descriptions and statements such as low tar/nicotine, no tar/nicotine, light, mild, moderate, natural, organic, no additives, no flavors
5. References to social status, elegance, femininity, masculinity, or weight loss.
In addition, the following restrictions apply to packaging and labeling:
1. Packaging must not resemble a food or cosmetic product
2. Nothing can be written on the outer cellophane wrapper
3. If hypersensitivity constituents are used, this should be indicated on the label.
Manual
The packaging must include a manual, printed in Arabic or English, that contains the following information:
1. Instructions for use and storing
2. Device components and technical characteristics.
3. Potential adverse effects for consumers
4. Statement indicating that the product is not recommended for use by young people or non-smokers
5. Circumstances where the product must not be used and warnings mentioned in Section 5.2 of the Standard and any special warnings for any specific groups
6. Addiction and toxicity
The GCC GSO draft standard stipulates that the following sentence must be placed on the product’s packaging:
للبيع في دول الخليج العربي
(For sale in the countries of the Gulf Cooperation Council)

Registration Procedures
Importers and manufacturers of vape products in the UAE are required to comply with the Emirates Conformity Assessment Scheme (ECAS) requirements and obtain Conformity Certificates from the Ministry of Industry and Advanced Technology (MoIAT).
The certification types are divided into four categories: hardware devices, e-liquids, and RoHS. Please select the appropriate product type for your application. Below is a detailed breakdown of the costs and procedures involved.
Product Registration
According to cabinet resolution 5/2019 and the MoIAT’s technical requirements for regulated products, importers and manufacturers of devices must register with the MoIAT and obtain the Conformity Assessment Certificate.
Hardware registration under ECAS is not a vape-specific certification, but rather a compliance pathway under MoIAT’s electrical safety and battery safety category, applied to all portable electronic devices.
Application Process
1. Login or create an account on the digital services platform MoIAT
2. Select the service: Issuing Conformity Certificates for Products According to Health and Safety Requirements
3. Upload all required documents, including product-related test reports and declarations
4. Pay the applicable fees to complete the submission process
Document Requirements
According to UAE.S 5030:2018 – Section 4.1.9, it is not permitted to import, manufacture, or place electronic nicotine products and cartridges on the UAE market without official approval from ESMA. The following documents must be submitted:
1. Safety test reports including drop test, leakage test, contact temperature, and electromagnetic compatibility, issued by scientific entities or laboratories accepted by ESMA
2. Complete list of ingredients in descending order by weight, indicating trade name, type, quantity, purpose of use, and emission levels
3. Toxicological studies of ingredients before and after use, including emissions when heated, issued by ESMA-accepted entities
4. Data on nicotine dose and absorption under normal or reasonably expected conditions
5. Written acknowledgment that the manufacturer and importer assume full responsibility for product quality and safety
6. Description of product usage and refill mechanism
7. Description of production process sequence, confirming conformity with the Standard
8. Stability study supporting the indicated shelf life
9. Laboratory reports from ESMA-accepted entities covering nicotine concentration, product quality, post-marketing analysis, and electrical safety
10. Risk assessment of the consumable component of the product
Additional documents required under the ECAS registration system (MOIAT / ESMA):
Declaration of Conformity (DoC) – Official statement affirming compliance with relevant UAE regulations
ECAS or G-Mark Certificate – One of these conformity certificates is required, depending on the product type
Barcode / GTIN – Mandatory for certain product categories during registration
Label and Packaging Design Files – Submitted for compliance review with UAE labeling and packaging requirements
Bilingual Instructions or Label Content – Arabic and English versions required for user manuals and safety information
Product Images – Clear photos showing the device, cartridge, and retail packaging
Company Documents – Valid trade license and manufacturing facility certificates; ISO or GMP certifications may be required for audit
Authorization Letter (if applicable) – Required if the applicant is not the original manufacturer or brand owner
The specific procedure can be referred to in the MoIAT Service Guide.
Processing Time
The application process for the conformity certificate typically takes 1.5 working days. However, the exact processing time may vary based on the application volume and other factors.
If the vape device contains e-liquid, you also need to prepare e-liquid-related documents. Please refer to the e-liquid requirements below.
RoHS Registration
Hardware equipment also requires a RoHS assessment application, which typically takes 1.5 working days.
Required Documents
1. A UAE industry/trade license stating the legal status of the applicant organization
2. Manufacturer declaration of conformity
3. Importer declaration of conformity
4. All test reports issued by laboratories according to the international standard ISO/IEC 17025:2017
Annual Maintenance and Information Update
Certificates issued under the ECAS system are valid for one (1) year. Renewal of the certificate requires the submission of updated technical documents and conformity assessment reports to ensure continued compliance with the applicable UAE regulations and standards.
Any changes to certified product specifications, manufacturing site, brand ownership, or applicant information must be communicated to the Conformity Assessment Body (CAB) and undergo re-evaluation and approval via the MoIAT platform, in accordance with the Procedure for Reviewing and Evaluating Applications for ECAS/EQM Certification (CAPRO-01).
How much does it cost to Register Vape Products in the UAE?
For manufacturers and importers registering vaping products and related components under the UAE Conformity Assessment System (ECAS), the Ministry of Industry and Advanced Technology (MoIAT) applies a standard fee structure for each product type (e-liquids, devices, labels, and RoHS).
As of 2025, the official fees are as follows:
- AED 600 for submitting a registration application
- AED 620 for the technical review of documents
- AED 500 for the issuance of a conformity certificate
In total, the basic cost per product registration is AED 1,720, excluding VAT and optional technical assessments. For applications that require site evaluation or technical assessor visits, an additional fee of AED 2,500 per working day applies. Any travel or logistical expenses incurred must also be covered by the applicant.
It is important to note that some third-party platforms may present simplified or combined pricing structures, but official registration must ultimately comply with the MoIAT fee schedule.
How Long Does it Take to Register Vape Products in the UAE?
The duration for obtaining approval for e-cigarette products in Dubai and the UAE can vary considerably. This process is influenced by factors such as the completeness of the application, the type of product, and the efficiency of the regulatory bodies involved.
General Approval Timelines:
Category | Shortest | Longest |
E-liquids | 4 weeks | 6 weeks |
Basic devices | 6 weeks | 8 weeks |
Advanced devices | 8 weeks | 12 weeks+ |
To ensure a smooth approval process, it is crucial to submit comprehensive documentation and adhere to all regulatory guidelines. Employing a consultant experienced with local regulations can further expedite the process.
How Much Tax Is Applied to E-Cigarettes in the UAE?
In the UAE, e-cigarettes and related products are classified as excise goods under the GCC Unified Agreement on Excise Taxation, and are subject to a 100% excise tax. In addition, all imports face a 100% customs duty, and a 5% value-added tax (VAT) is applied at the retail level.
To ensure tax compliance, all nicotine-containing products must carry Digital Tax Stamps (DTS) issued by the Federal Tax Authority (FTA). Companies must register with the FTA and apply for DTS through the official portal before importing or distributing products in the UAE.
How Can Hangen Help You Start Your Business?
1. End-to-End E-Liquid Solutions: Hangsen provides comprehensive support across multiple stages, including identifying product requirements, flavor development, laboratory testing and analysis, packaging design, and distribution. Achieving customer satisfaction is our ultimate goal.
2. Flavor Customization: Hangsen boasts significant expertise in custom product development and has maintained long-term partnerships with the world's leading vaping brands. This collaboration enables us to effortlessly craft flavor formulas that satisfy the preferences of Middle Eastern consumers.
3. Regulatory Compliance: Hangsen provides expert guidance on adhering to the specific legal standards of Middle Eastern countries. Our services include understanding and complying with regional regulations on product safety, labeling, packaging, and distribution. We assist clients in preparing the necessary documentation and ensuring all legal requirements are met, which is crucial for successful market entry and operation. With numerous successful client case studies, our expertise in navigating these complex regulatory landscapes has proven effective.
4. Logistics Support: We maintain deep cooperation with the most professional logistics service providers in the Middle East, offering door-to-door delivery support to ensure a smooth operation of the backend supply chain. This collaboration enables a true closed-loop service experience.
Learn More
Middle East - An Emerging Vaping Market with Unlimited Potential
Hangsen Highlights Innovative E-liquid Solutions at World Vape Show 2024 in Dubai, UAE
Bar Salt White Label E-liquid Solution
Middle East E-Liquid Flavor Collection
Frozen Summer Series: Chilling Out with Hangsen's Refreshing Fruit Flavors
HANGSEN INTERNATIONAL GROUP
Hangsen is a world-leading e-liquid manufacturer, offering custom flavor solutions for vape devices and premium white-label e-liquid services. Our e-liquids are renowned for their high consistency, stability, safety, and regulatory compliance. With business operations spanning over 80 countries and regions worldwide, Hangsen continues to set industry standards.
Contact Us
If you are interested in our Compliance Consulting Services, please feel free to contact us.